Quantitation of nickel
| Basic experiment | Author(s): Monika Partsch (Technische Universität München - AuTUM) |
| Chemical | Formula (Hill) | Safety | Amount | |
|---|---|---|---|---|
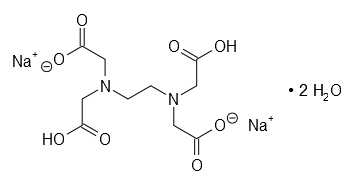 | EDTA disodium salt dihydrate | C10H14N2Na2O8·2 H2O | 10 g | |
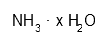 | Ammonia solution 25% | H3N | H314 H335 H400 P261 P273 P280 P305 + 351 + 338 P310 | 30 mL |
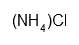 | Ammonium chloride | ClH4N | H302 H319 P305 + 351 + 338 | 10 g |
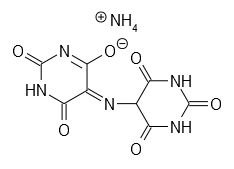 | Murexide | C8H8N6O6 | ? | |
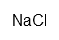 | Sodium chloride | ClNa | ? | |
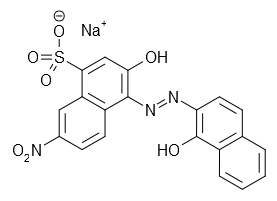 | Eriochrome black T | C20H12N3NaO7S | ? | |
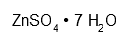 | Zinc sulfate heptahydrate | O4SZn·7 H2O | H302 H318 H410 P273 P280 P305 + 351 + 338 P501 | ? |
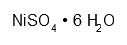 | Nickel sulfate hexahydrate | NiO4S·6 H2O | H302 + 332 H315 H317 H334 H341 H350i H360D H372 H410 P201 P261 P273 P280 P308 + 313 P501 | 56 g |
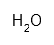 | Water distilled | H2O | ca. 1000 mL | |