Quantitation of copper(II) ions
| Basic experiment | Author(s): Monika Partsch (Technische Universität München - AuTUM) |
| Chemical | Formula (Hill) | Safety | Amount | |
|---|---|---|---|---|
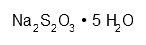 | Sodium thiosulfate pentahydrate | Na2O3S2·5 H2O | ca. 15 g | |
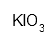 | Potassium iodate dried at 110°C | IKO3 | H272 H315 H319 H335 P220 P261 P305 + 351 + 338 | ca. 1 g |
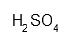 | Sulfuric acid 2 mol/L | H2O4S | H314 H290 P280 P301 + 330 + 331 P305 + 351 + 338 P309 + 310 | 250 mL |
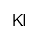 | Potassium iodide | IK | H302 H315 H319 P305 + 351 + 338 | ca. 20 g |
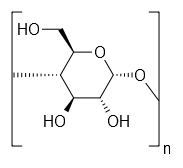 | Starch solution w(starch)=1% | (C6H10O5)nH2O | 20 mL | |
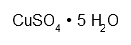 | Copper sulphate pentahydrate | CuH10O9S | H302 H315 H319 H410 P273 P305 + 351 + 338 P501 | ca. 3.12 g |
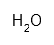 | Water | H2O | ? | |